MRI is a bioelectric mapping device
The forgotten history of one of medicine's most important machines
A fundamental problem that many in the bioelectricity field are trying to solve is the ability to image bioelectric patterns in deep tissue. We believe this problem is already solved.
From the start, the MRI was a bioelectricity-mapping device.
We all know that the MRI is one of the most important medical devices ever made. How many of us who know of the impact that the MRI has had on cancer and other diagnostics could answer the questions: How does it work? And How did we know to make it that way?
For this reason, I will start with the
Historical Context
MRI uses a strong magnet to align hydrogen atoms in the body’s water. Radio waves then disrupt this alignment, causing the atoms to tip over. As they return to their original alignment, they emit signals. These signals differ based on nearby tissues and ion levels, which affect water structure and relaxation times, called T1 and T2. T1 is the time it takes for protons to realign with the magnetic field; T2 is the time it takes for the emitted signal to fade. These measurements help create detailed images of cellular states.
The MRI was originally created by Raymond Damadian to be a machine that could differentiate cancer tissue from healthy tissue based on a realization that electrical properties of the cells correlated with water dynamics. Damadian, in his 1971 paper proposing the MRI, stated,
My own experiments with Escherichia coli suggested that altered selectivity coefficients of alkali cations in biologic tissue, such as occur in neoplastic tissue, can indicate alterations in tissue water structure.
Here, alkali cations are sodium and potassium, the prime charge carriers in bioelectricity, and tissue water structure changes are observable by the proposed MRI device.
The idea was conceived from Saint Louis, MO where Damadian was doing postdoctoral studies as a fellow at the Washington University School of Medicine. He was studying the kidney, which he considered to be the organ that regulates bioelectricity. Damadian conceived the idea after being made aware of the work of Dr. Gilbert Ling, and his Association-Induction Hypothesis prompting him to realize that variations in intracellular ion and protein interactions could alter the relaxation times of the protons of water molecules, which could be measured by NMR to provide a non-invasive cancer screening method. I wrote about Gilbert Ling and his alternative theory of the cell extensively in a past post you can checkout to learn more.
Understanding the profound but disputed significance of Ling’s hypothesis in medicine, Damadian became one of Ling’s key proponents, stepping in to personally support Ling’s research when traditional sources withdrew due to mainstream doubt.
After all, they had something in common: they had both been ostracized from the broader scientific community for their radically counter-narrative ideas. It is arguable that Damadian himself was snubbed in the awarding of a Nobel Prize for his creation of the MRI.1
The Nobel Prize can be split among up to three recipients; however the award for the creation of the MRI was only split between two parties with neither being Damadian.
In 2003, a group called Friends of Raymond Damadian organized protests via full-page newspaper advertisements and a letter-writing campaign to the Nobel Committee. The campaign spent over $1.2 million. Advertisements quoted endorsements from a number of prominent scientists.
Damadian was a vocal creationist, believing that God created the heavens and the Earth in 6 days. Damadian himself eventually was convinced, despite being hesitant, that this was the reason he was snubbed the prize. Anyway, this is another rabbit hole you are welcome to explore yourself. Here is an entry point.
The project to build the MRI took 8 years and cost $2M(approximately $10.8 million in 2025 dollars according to a conventional CPI calc). It was repeatedly stalled due to lack of funding for the idea that a machine using fields would have any chance of reading the state of cells in a live organism to lead to meaningful conclusions. Eventually, Damadian was able to get buy-in from investors to found FONAR, a private company, in order to finally get the resources he needed to finish the project.


Damadian always envisioned his machine to be something that would both be able to READ the state of the body and WRITE to correct it when necessary.
However, after the unveiling of his first commercial MRI, larger biomedical device manufacturers such as Siemens and General Electric directly infringed on Damadian’s patent and started copying the machine to sell their own versions. After a 5-year legal battle, Damadian was vindicated with a $128M settlement from GE among others.
With this background, let’s return to how
The MRI solves a new, old problem
Bioelectricity research is more popular than ever. One of the biggest problems researchers in both industry and academic settings working in this space have is figuring out how to get bioelectric information in the body of an organism larger than a petri dish in real time, because the methods used in cells and small organisms will not scale to something like a mouse, much less, a human.
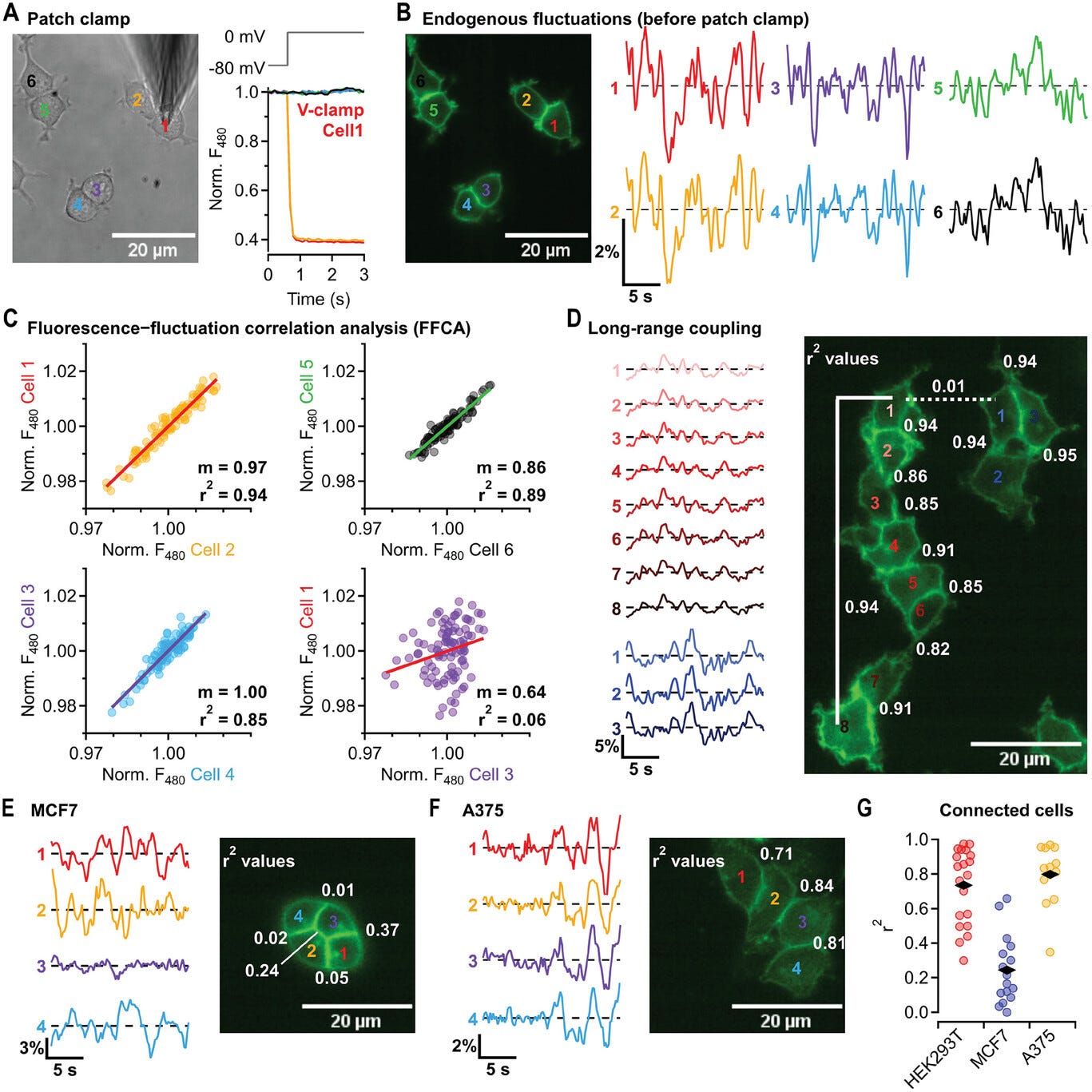
The way this is done now other than single-cell patch clamp is via special dyes like DiBAC4(3) and proteins called genetically encoded voltage indicators (GEVIs). The way that these work is by using a light typically inside a microscope to excite the dye or the protein with energy that then leads to a return signal that can be picked up and recorded as a given fluorescent intensity. Changes in this fluorescent intensity can be calibrated to get an idea of absolute membrane potential from this signal.
This doesn’t translate to deep tissue imaging because the way we excite the indicator with light and then get a return response just isn’t workable in the larger 3D spatial environment of the body due to optical scattering and absorption.
So what does the state-of-the-art for deep tissue bioelectric imaging look like?
This problem is very important in neuroscience because the paradigm of having bioelectric signals playing a causal role in underlying cellular states or higher-level disease states is more precedented. The current state of the art in this space is multiphoton microscopy combined with advanced GEVIs, such as in DEEPscope systems that integrate two-photon and three-photon techniques for imaging neural activity at depths up to several millimeters, alongside non-optical methods like functional ultrasound (fUS) for indirect hemodynamic (blood flow) correlates of bioelectric activity and electrophysiological source imaging from EEG or MEG data to reconstruct electric field networks throughout the brain volume.
Limitations of this approach are penetration depth (typically 1-2 mm for optical methods due to tissue scattering), low signal-to-noise ratios for subtle voltage fluctuations, photobleaching and phototoxicity from prolonged excitation, detector sensitivity constraints, channel crosstalk in multi-indicator setups, and the invasive nature required for truly deep or whole-brain coverage.
Despite this, for imaging the bioelectrics of non-excitable cells, some groups that care about this problem at all are working to adapt the above-mentioned tools for the slower, more minuscule changes in this setting, such as using ultrasensitive GEVIs like those that reveal oscillatory membrane potentials in cell lines like HEK293, enabling detection of non-action-potential dynamics in multicellular contexts. For example, a neuron might have a 60 mV action potential on the order of milliseconds while a less specialized cell will have a 10-20 mV change over the course of minutes or hours.
Another cutting-edge approach entails genetically modifying organisms from the embryonic stage in order to have them express a version of a GEVI, however, instead of being excited by light and returning light, the intent is for these indicators to work by exciting the protein with near-infrared light to create a 3D spatio-temporal map with improved depth penetration. I understand there to be similar attempts to progress using X-rays but nothing on this is published yet.
A while back, already believing it to be the case that the MRI would act as a turnkey solution to this problem we’ve highlighted yet looking for authoritative sources to back this claim, I came across an October 2024 pre-print for a paper called Detection of changes in membrane potential by magnetic resonance imaging. In it, they even had the line,
To the authors’ knowledge, this is the first report that changes in membrane potential can be detected through MRI, which has the advantage of noninvasively acquiring signals over a large area with good spatial resolution.
underscoring the overlooked legacy. Yet, when the peer-reviewed version, published in July 2025 came out with a retitled “Responses to membrane potential-modulating ionic solutions measured by magnetic resonance imaging of cultured cells and in vivo rat cortex,” the line was removed entirely.
Reviewers also got rid of all references to direct neuronal detection2 and claims that T2/MT “directly reflect changes in membrane potential,” further muting the implications of the study.
With all this said, it is clear this is a problem that is both important enough that bleeding-edge groups are working to solve it while at the same time, having a relatively turnkey solution that has existed for decades the scientific community seems to have forgotten about.
Our goal at AION is to reclaim the sidelined legacy amid persistent amnesia, and restore the MRI as its intended foundation: a device that reads and writes cellular states 100% non-invasively.
We are actively seeking adaptable engineers aligned with our mission of advancing human longevity to develop hardware at the intersection of acoustics, magnetism, and RF. Interested folks can learn more by reading our whitepaper.
As much as we innovate, we remember.
-Benjamin Anderson
Nostr: ben@buildtall.com
Likely due to the retraction of a paper called ‘In vivo direct imaging of neuronal activity at high temporospatial resolution’ that was heavily leaned on to build the argument for neuronal imaging implications. The paper was retracted following an editorial expression of concern over methods described in the paper being inadequate to allow reproduction of the results.